Return
1. Underground bedded salt deposits have thicknesses up to 3000 feet.
2. Are often found at 500 to 2,000 feet below ground surface.
3. Can contain significant impurities.
4. Are found in layers interspersed with materials such as anhydrite, shale, and other more permeable salts (eg., potassium chloride)
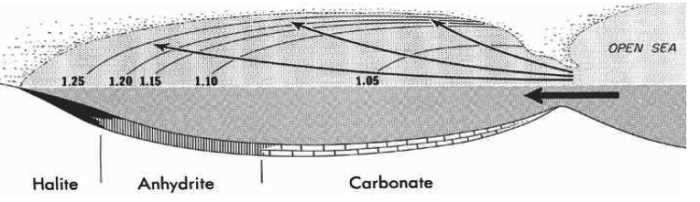
"Although all water bodies on the surface and in aquifers contain dissolved salts, the water must evaporate into the atmosphere for the minerals to precipitate. For this to happen, the water body must enter a restricted environment where water input into this environment remains below the net rate of evaporation. This is usually an arid environment with a small basin fed by a limited input of water. When evaporation occurs, the remaining water is enriched in salts, and they precipitate when the water becomes supersaturated."..."When scientists evaporate ocean water in a laboratory, the minerals are deposited in a defined order that was first demonstrated by Usiglio in 1884. The first phase of the experiment begins when about 50% of the original water depth remains. At this point, minor carbonates begin to form. The next phase in the sequence comes when the experiment is left with about 20% of its original level. At this point, the mineral gypsum begins to form, which is then followed by halite at 10%." (from Evaporite - Wikipedia.htm) Evaporation of about 80 feet of sea water is required to produce a foot of salt.
Salt deposits found in Kansas are as shown below.
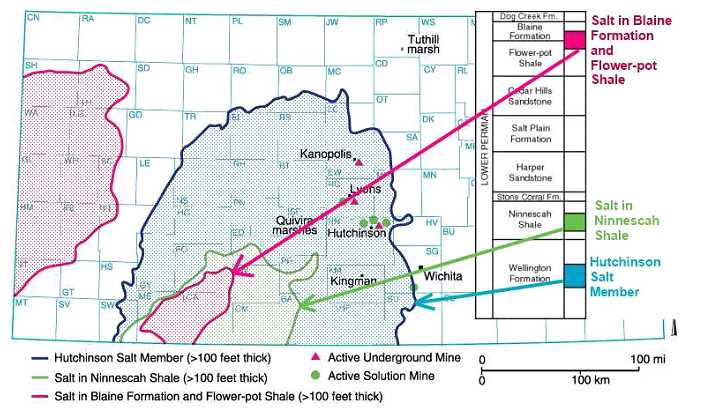
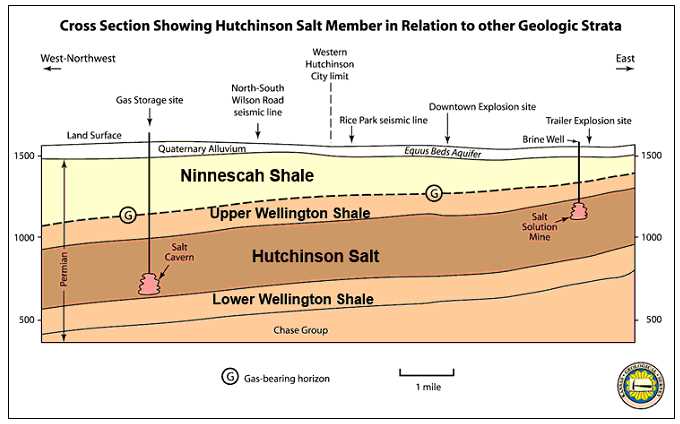
"The eastern edge of the Hutchinson Salt Member is actively being eroded, or dissolved, by contact with ground water. This area, where the salt is closest to the surface, is known as the dissolution front. Because salt is so easily dissolved in water, outcrops at the surface are not present in Kansas." (illustration from KGS--Hutchinson Response--General Geology.htm)
"The Michigan Basin is one of the greatest areas of rock salt and other evaporites like gypsum in the world, and these sediments have been the basis of major chemical and plasterboard industries in Michigan. This basin was bounded on the northwest by the Wisconsin Arch and on the southeast by the Cincinnati Arch. Also the Kankakee Arch developed across northeastern Illinois, forming two separate basins. Although it did not form a land barrier, it greatly restricted circulation of sea waters within the Michigan Basin.
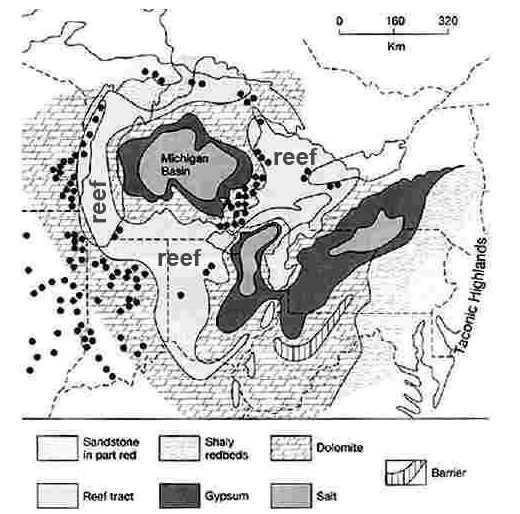
The rate of subsidence within the Michigan Basin then increased markedly, and a series of coral reefs developed along the Kankakee Arch and in the shallow waters along the western and southern margins of the basin. Some of these reefs can be seen today on the south side of Chicago, and I-94 crosses over a giant quarry into one of them. Assuming that these reefs formed in an environment similar to modern reefs, we can infer something about the Great Lakes area during this time. All modern reefs grow on a shallow bottom, no deeper than the depth of light penetration, generally about 200 feet (60 m). Furthermore, coral reefs are restricted to tropical regions where water temperatures are consistently above 77oF. Thus, the presence of extensive reefs around the Michigan Basin suggests a shallow tropical or sub-tropical environment. Well preserved fossils in most of these ancient reefs substantiate this assumption. Outward from the reefs, in the deeper water, muds rich in CaCO3 accumulated on the floor of the vast shallow seas, generally producing thinly bedded dolomite that contains few fossils. But the genial climates came to an end.
The close of the period was a long time of aridity in which the seas became so salty that living creatures swam to a more suitable home. The conditions were roughly analogous to the present-day Mediterranean Sea where water from the Atlantic flows in over the rather shallow restriction at the Straits of Gibraltar. The arid climate in the region evaporates the sea water and concentrates the salt. The heavier, highly saline water sinks to the bottom and cannot escape and therefore tends to accumulate. Great salt beds (named the Salina Salt Beds) were deposited in the basin. In the central part of the basin 2300 feet of alternating salt, shale, and limestone beds have been penetrated in the Salina, with 1600 feet of rock salt. The rock salt bowl becomes thinner towards its rim. Most gypsum units are less than 20 feet in thickness. Contrast that with the over 400 feet of halite in the basin. These deposits are buried up to 1250 feet below the present surface.
The extensive development of offshore carbonate rocks and an absence of sandstones and shales suggests that no land mass was present in the Great Lakes area while these deposits were being formed." (from geo.msu.edu/geogmich/evaporite.html 8 Feb 2009.)
Visualize a subsiding, circular basin in lower Michigan, surrounded by shallow waters and coral reefs. At that time the Great Lakes area was near the equator, and as a result, there was extensive evaporation of sea water. To make up for this, salt water was constantly being added to the Michigan (evaporation) Basin through shallow passages in the reefs. Waters in the basin were therefore saltier than in the "open ocean" outside of the basin. Since the higher salt concentration caused the water to become heavier, the highly saline water settled to the bottom of the basin rather than mixing with the inflowing fresher waters from the surrounding seas. In this way, salt concentrations in the Michigan Basin grew, until gypsum (CaSO4•2H2O), anhydrite (CaSO4), and finally rock salt (NaCl) were precipitated on the underlying carbonates. An aggregate thickness of 600 m of gypsum and salt accumulated in the basin, with one bed of salt nearly 150 m thick. It would take a column of sea water nearly 1000 km deep to form a layer of salt 600 m thick. Great thicknesses of anhydrite and gypsum are also present.
The extensive development of offshore carbonate rocks and an absence of sandstones and shales suggests that no land mass was present in the Great Lakes area while these deposits were being formed. The end of Mississippian epoch was a time of shallowing seas in Michigan, and of warm and often arid climates, such that the sea deposits were limestones, shales, and gypsum. Along the shores of the old seas waves and currents heaped piles of sand just to the water level, sand bars developed. Today in drilling for oil we find those old bars and discover that in many of them natural gas has collected.
There is no evidence of volcanic activity in either the Kansas Hutchinson Salt Member area or the Michigan Basin ! A number of kimberlites are known to exist in Riley and Marshall counties of northeastern Kansas. Unlike volcanoes, kimberlites do not pour out lava. Instead, they explode violently to the surface, forming small craters and leaving behind a mixture of igneous and sedimentary rock that often contains garnets, a dark red semi-precious stone. (Kansas Geological Survey, Oct. 29, 1999) (from geo.msu.edu/ geogmich/evaporite.html 8 Feb 2009.)
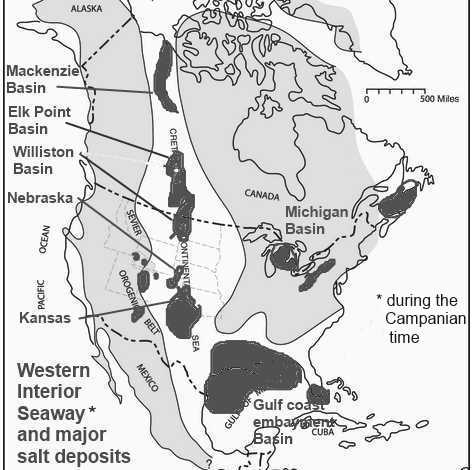
As you can see many of the major salt deposits in USA and Canada are located where in the past there was a shallow Western Interior Seaway of the Cretaceous time. "These deposits occurred in various geologic eras and all of them (in Canada) are the remains of ancient inland seas. The shorelines of these ancient seas, which outline the edges of the salt beds mark the occurrences of the oil, gas and coal deposits which have been found in such abundance in Canada." (Salt in Canada) The western interion seaway was present at a different time then when the Michigan Basin was in existance. "The Western Interior Seaway (also called the Cretaceous Seaway, the Niobraran Sea, the North American Inland Sea, and the Western Interior Sea) was a large inland sea that existed during the mid- to late Cretaceous period as well as the very early Paleogene," (Wikipedia-Western Interior Seaway) And these salt layers are surrounded by shales. By geologic time scale dating, in the Michigan area "during the Cambrian and Ordovician periods, one rather elongated basin extended from Missouri northeastward through Illinois and lower Michigan. This basin was bounded on the northwest by the Wisconsin Arch and on the southeast by the Cincinnati Arch. During the middle Silurian the Kankakee Arch developed across northeastern Illinois, forming two separate basins. Although it did not form a land barrier, it greatly restricted circulation of sea waters within the Michigan Basin." (from geo.msu.edu /geogmich/ evaporite.html 8 Feb 2009.) The Michigan salt formation is bounded by coral reefs. By the gegologic time scale concept their formations were separated in time by up to 300 million years. (The Louann Salt is a widespread evaporite formation that formed in the Gulf of Mexico during the Callovian in the mid Jurassic and will not be discussed.)
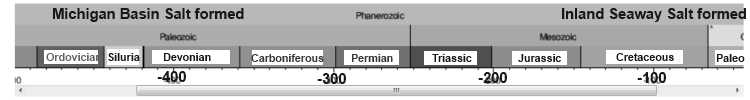
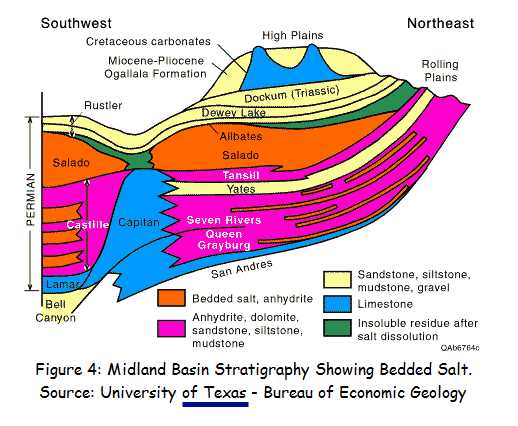
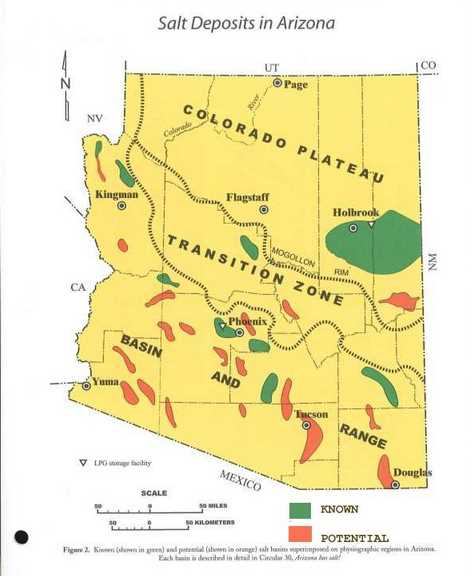
The very presence of bedded salt in the geologic record is evidence that it was not deposited during a WET "global flood" year (371 days). Salt dissolves in water, it doesn’t deposit out of water unless the water is saturated with salt. And when it is saturated with salt, no vertebrate life can live in those waters. So, why do we find huge salt beds in the middle of the sedimentary rock formations, which are very widespread and in some locations stacked one above another as discussed above? How did this happen in the midst of the biggest water event in history—the Noachian flood? Obviously it didn't !! And as per Genesis 1:2 "Thus the heavens and the earth were finished, and all the host of them." (ESV) it is obvious that many of these salt formations are pre-Flood !! And thus covered to varied depths by other materials.